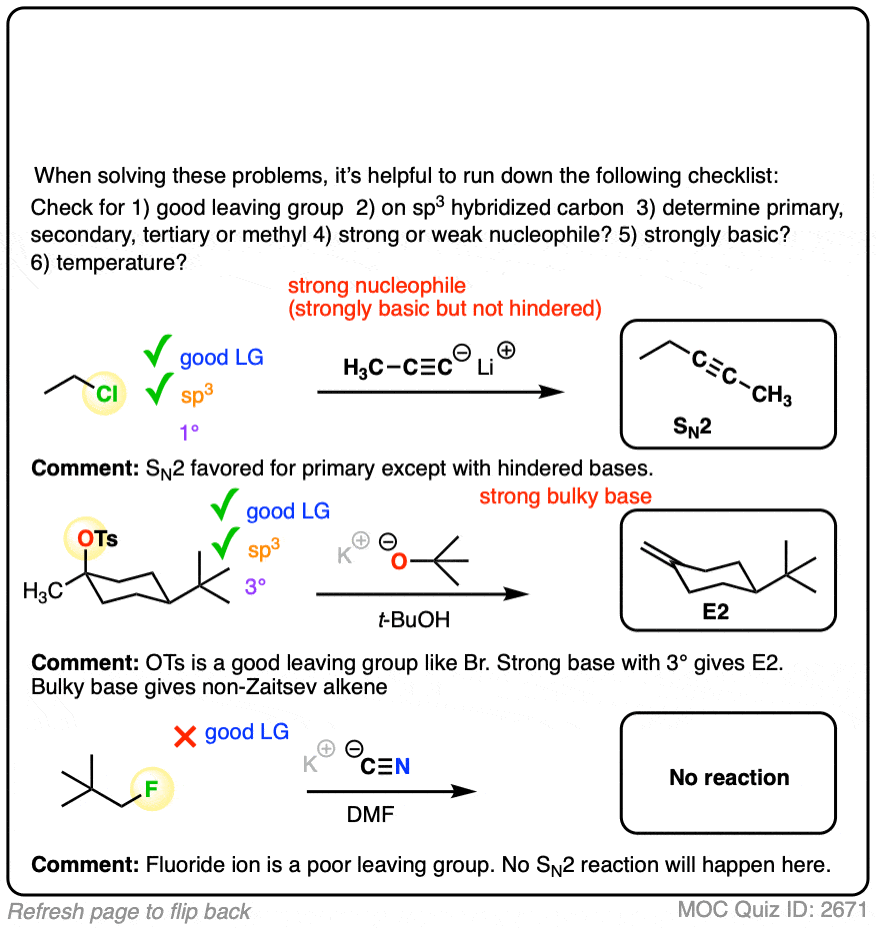
Organic chemistry is a fascinating field that explores the behavior and interactions of carbon-based compounds. Among the many concepts in this discipline, nucleophilic substitution and elimination reactions are fundamental to understanding how molecules react and transform. These reactions are classified into different mechanisms—SN1, SN2, E1, and E2—each with unique characteristics and conditions under which they occur. Whether you're a student preparing for an exam or a researcher delving into chemical synthesis, mastering these mechanisms is essential.
The competition between SN1, SN2, E1, and E2 reactions often determines the outcome of a chemical reaction. For instance, SN1 and SN2 are types of nucleophilic substitution reactions, while E1 and E2 are elimination reactions. The choice of mechanism depends on several factors, including the structure of the substrate, the strength of the nucleophile or base, the solvent used, and the temperature of the reaction. Understanding these factors helps predict the major product formed in a given reaction.
This article will guide you through the key principles of SN1, SN2, E1, and E2 reactions, explaining their mechanisms, conditions, and applications. We'll explore how each reaction operates, what influences its occurrence, and how to identify the correct mechanism for a given scenario. Additionally, we’ll provide insights into the stereochemistry and regiochemistry involved in these reactions, ensuring a comprehensive understanding of organic chemistry fundamentals. Whether you're looking to solve practice problems or deepen your knowledge, this resource is designed to support your learning journey.
Understanding Nucleophilic Substitution Reactions: SN1 vs SN2
Nucleophilic substitution reactions involve the replacement of a leaving group in a molecule by a nucleophile. Two main mechanisms govern these reactions: SN1 (Substitution Nucleophilic Unimolecular) and SN2 (Substitution Nucleophilic Bimolecular). Each has distinct features that influence the reaction pathway.
SN1 Mechanism:
The SN1 mechanism involves two steps: the formation of a carbocation intermediate and the attack by the nucleophile. This mechanism is typically observed in reactions involving tertiary substrates due to the stability of the resulting carbocation. The rate of the reaction depends only on the concentration of the substrate, making it a first-order reaction. SN1 reactions are common in polar protic solvents, where the solvent can stabilize the carbocation intermediate.
SN2 Mechanism:
In contrast, the SN2 mechanism occurs in a single step, where the nucleophile attacks the substrate from the opposite side of the leaving group, leading to an inversion of configuration. This mechanism is favored by primary substrates, as the steric hindrance is minimal. The rate of the reaction depends on the concentrations of both the substrate and the nucleophile, making it a second-order reaction. SN2 reactions typically occur in polar aprotic solvents, which do not stabilize the transition state but allow for efficient nucleophilic attack.
Understanding the differences between SN1 and SN2 reactions is crucial for predicting the outcome of a chemical reaction. Factors such as the nature of the substrate, the strength of the nucleophile, and the solvent play significant roles in determining which mechanism dominates.
Elimination Reactions: E1 and E2 Mechanisms
Elimination reactions involve the removal of a small molecule, such as water or hydrogen halide, from a larger molecule, resulting in the formation of a double bond. Two primary mechanisms govern these reactions: E1 (Elimination Unimolecular) and E2 (Elimination Bimolecular).
E1 Mechanism:
The E1 mechanism also proceeds in two steps, similar to the SN1 mechanism. The first step involves the formation of a carbocation intermediate, followed by the loss of a proton to form a double bond. This mechanism is typically observed in reactions involving tertiary substrates and is influenced by the stability of the carbocation. The rate of the reaction depends only on the concentration of the substrate, making it a first-order reaction. E1 reactions are commonly seen in polar protic solvents, where the solvent can stabilize the carbocation intermediate.
E2 Mechanism:
The E2 mechanism occurs in a single step, where the base abstracts a proton from the substrate while the leaving group departs, leading to the formation of a double bond. This mechanism is favored by secondary and tertiary substrates, as the steric hindrance is less compared to primary substrates. The rate of the reaction depends on the concentrations of both the substrate and the base, making it a second-order reaction. E2 reactions typically occur in polar aprotic solvents, which do not stabilize the transition state but allow for efficient base attack.
The choice between E1 and E2 mechanisms is influenced by several factors, including the structure of the substrate, the strength of the base, and the solvent used. Understanding these mechanisms is essential for predicting the outcome of elimination reactions in organic chemistry.
Choosing the Correct Mechanism: SN1, SN2, E1, or E2
Selecting the appropriate mechanism for a given reaction is a critical skill in organic chemistry. Several factors influence whether a reaction follows an SN1, SN2, E1, or E2 pathway. These include the structure of the substrate, the nature of the nucleophile or base, the solvent, and the reaction conditions.
Substrate Structure:
The type of alkyl halide plays a significant role in determining the mechanism. Primary substrates favor SN2 reactions due to minimal steric hindrance, while tertiary substrates are more likely to undergo SN1 or E1 mechanisms because of the stability of the resulting carbocation. Secondary substrates can follow either SN1 or SN2, depending on other factors.
Nucleophile/Base Strength:
Strong nucleophiles favor SN2 reactions, while strong bases are more likely to promote E2 reactions. The solvent also affects the reaction mechanism; polar protic solvents stabilize carbocations and are suitable for SN1 and E1 reactions, whereas polar aprotic solvents are ideal for SN2 and E2 reactions.
Temperature and Solvent Effects:
Higher temperatures generally favor elimination reactions (E1 and E2), as they provide the necessary energy for the formation of the double bond. The solvent's polarity and ability to stabilize intermediates or transition states also play a crucial role in determining the mechanism.
By considering these factors, chemists can predict the most likely mechanism for a given reaction and design experiments accordingly. This understanding is essential for both academic and industrial applications in organic chemistry.
Stereochemistry and Regiochemistry in Organic Reactions
Stereochemistry and regiochemistry are essential aspects of organic reactions, influencing the outcome and properties of the products formed. These concepts describe the spatial arrangement of atoms and the specific positions where reactions occur, respectively.
Stereochemistry:
Stereochemistry refers to the three-dimensional arrangement of atoms in a molecule. In reactions like SN2, the nucleophile attacks the substrate from the opposite side of the leaving group, leading to an inversion of configuration. This phenomenon is known as Walden inversion. In contrast, SN1 reactions often result in racemization due to the formation of a planar carbocation intermediate, allowing the nucleophile to attack from either side.
Regiochemistry:
Regiochemistry deals with the specific position where a reaction occurs on a molecule. In elimination reactions, such as E2, the base abstracts a proton from a specific position, leading to the formation of a double bond at a particular location. Zaitsev's rule states that the more substituted alkene is the major product in elimination reactions, as it is more stable. However, in some cases, the less substituted alkene (Hofmann product) may be favored if the base is bulky.
Understanding stereochemistry and regiochemistry is crucial for predicting the major product of a reaction and designing synthetic pathways. These concepts help chemists control the outcome of reactions and achieve desired products with specific structures and properties.
Practical Applications and Examples of SN1, SN2, E1, and E2 Reactions
The practical applications of SN1, SN2, E1, and E2 reactions are vast and span various fields, including pharmaceuticals, materials science, and environmental chemistry. These reactions are fundamental in the synthesis of complex molecules and the development of new drugs.
Pharmaceutical Industry:
In the pharmaceutical industry, SN2 reactions are commonly used to synthesize chiral drugs, where the stereochemistry of the product is crucial for its biological activity. For example, the synthesis of certain antibiotics involves SN2 mechanisms to ensure the correct configuration of the active compound. On the other hand, E1 and E2 reactions are employed in the formation of unsaturated compounds, which are essential in drug development.
Materials Science:
In materials science, elimination reactions are used to create polymers and other materials with specific properties. For instance, the production of polyethylene involves the elimination of small molecules during the polymerization process. Similarly, SN1 reactions are utilized in the synthesis of various monomers, which are then polymerized to form plastics and other materials.
Environmental Chemistry:
In environmental chemistry, understanding these reactions helps in the degradation of pollutants. For example, the breakdown of certain organic compounds in the environment can occur via SN1 or E1 mechanisms, depending on the conditions. This knowledge is vital for developing strategies to mitigate pollution and manage waste effectively.
By exploring these practical applications, it becomes evident that mastering the mechanisms of SN1, SN2, E1, and E2 reactions is not only academically important but also has significant real-world implications. These reactions continue to shape the way we approach chemical synthesis and environmental management in various industries.

